With TZIELD, it’s possible to take control of what's next
TZIELD can help you or your loved one by slowing down the onset of insulin-dependent type 1 diabetes.

TZIELD is changing the possibilities of autoimmune type 1 diabetes (T1D) treatment
Managing T1D is no longer just about waiting until you experience symptoms. It's possible to take control of what's next. TZIELD could mean more time without the need for insulin injections, more time to learn about eventual symptoms and risks, and more time to get ready for daily insulin management.
The effectiveness of TZIELD
Time to onset of stage 3 type 1 diabetes
With a single 2-week course of TZIELD, people had a median of 4 years* before the onset of insulin-dependent type 1 diabetes, compared to 2 years with placebo.
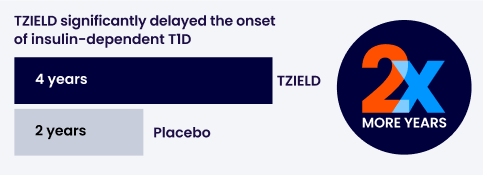
In a clinical study, the risk of onset of insulin-dependent type 1 diabetes was significantly lower for people who were given TZIELD, compared with people who were not given TZIELD.
*In a clinical trial with TZIELD, people had a median† of 4 years before the onset of insulin-dependent (Stage 3) type 1 diabetes, compared to about 2 years with placebo. Results may vary.
†Median is the middle number in a group of numbers arranged from lowest to highest
The clinical trial included 76 people in the stage right before they became insulin-dependent (Stage 2). 44 received TZIELD and 32 received a placebo. People were followed until they were diagnosed with insulin-dependent T1D.
The median amount of time people were followed was 51 months (just over 4 years). People not diagnosed with insulin dependence were followed for different periods of time.
TZIELD has been studied in clinical trials for over 10 years. See the TZIELD safety profile and Important Safety Information below and talk to your doctor and wider healthcare team for more information or medical advice about side effects.
There are 3 stages in type 1 diabetes
Early non-symptomatic stage
No Insulin Needed
- The immune system has started attacking beta cells
- 2+ autoantibodies present
- Blood sugar levels are within a normal range
Early non-symptomatic stage
No Insulin Needed
- Beta cells continue to be attacked
- 2+ autoantibodies present
- Blood sugar levels are outside of a normal range (dysglycemia)
Symptomatic stage
Insulin needed
- Beta cells are damaged and can’t make enough insulin to regulate blood sugar
- Autoantibodies may be present
- Blood sugar levels are higher than a normal range (hyperglycemia)
- Risk of hospitalizaton (diabetic ketoacidosis)

Want a deeper dive into the stages of type 1 diabetes?
Learn MoreWant to explore why delaying the onset of insulin-dependent T1D can be so important?
Learn MoreThe Safety Profile of TZIELD
Most common side effects
Rash36% of people
Decrease in white blood cell counts (Leukopenia)21% of people
Headache11% of people
These are not all the possible side effects of TZIELD. Talk to your doctor and healthcare team for more information or medical advice about side effects.
The clinical trial that explored the safety of TZIELD included 76 people who had Stage 2 T1D. 44 people in this trial were given TZIELD. 32 people in the trial were given placebo. The median† amount of time that people were followed in the trial was 51 months (just over 4 years). Results may vary
†Median is the middle number in a group of numbers arranged from lowest to highest.
Serious side effects
TZIELD may cause serious side effects, including:
Cytokine Release Syndrome (CRS)
2% of people
- fever
- muscle and joint pain
- headache
- feeling tired (fatigue)
- nausea
- increased liver enzymes in your blood
These signs and symptoms may start during the first 5 days of TZIELD treatment. Tell your doctor right away if you develop any signs or symptoms of CRS during treatment with TZIELD.
Decrease in White Blood Cells (Lymphopenia)
73% of people
TZIELD may cause a drop in a type of white blood cell called a lymphocyte. This can affect your body’s ability to fight infections. A decrease in white blood cell count can happen after your first dose of TZIELD, but will start to go back to normal after your fifth dose, and completely return to normal within 2 weeks of finishing treatment.
The safety of TZIELD was studied in 5 clinical trials. Side effects were monitored in 773 patients who were given TZIELD and 245 patients who were given placebo. Not all of the patients in these studies had Stage 2‡ type 1 diabetes.
‡Stage 2 type 1 diabetes means someone has tested positive for 2 or more type 1 diabetes-related autoantibodies and has abnormal blood sugar levels (called dysglycemia) but does not have noticeable symptoms. In this stage, type 1 diabetes is progressing, but insulin injections aren’t needed yet.
Haven’t been screened for autoimmune type 1 diabetes yet?
Consider talking to your or your loved one's doctor about autoantibody screening, a blood test different from annual blood work, to detect type 1 diabetes early. It's the first step you can take to determine if TZIELD may be right for you or your loved one.
Remember, type 1 diabetes doesn’t wait. Neither should you.
The window to treat with TZIELD can be short. Now is the time to take action and discuss with your doctor if TZIELD is right for you. Here are some additional resources to help you learn more.

The time is now to screen for type 1 diabetes
Because the window to treat with TZIELD can be short, take the first step and ask your doctor about autoantibody screening for autoimmune type 1 diabetes.
Important Safety Information and Approved Use
What is the most important information I should know about TZIELD? TZIELD may cause serious side effects. These include:- Cytokine release syndrome (CRS). Signs and symptoms may start during the first 5 days of TZIELD treatment and could include fever, nausea, feeling tired (fatigue), headache, muscle and joint pain, or increased liver enzymes in your blood. Tell your healthcare provider right away if you develop any signs and symptoms of CRS during treatment with TZIELD
- Decrease in white blood cells. TZIELD may cause a decrease in a type of white blood cell called lymphocytes. A decrease in white blood cells is a serious, but common side effect that can affect your body’s ability to fight infections. A decrease in white blood cell counts can happen after your first dose. Your white blood cell counts will start to go back to normal after your fifth dose of TZIELD. Some people may develop longer and more severe decreases in lymphocytes
Your healthcare provider will do blood tests to check your liver and your complete blood counts before you start treatment and during treatment with TZIELD. During and after your treatment with TZIELD, your healthcare provider will check for serious side effects, as well as other side effects, and treat you as needed. Your healthcare provider may temporarily or completely stop your treatment with TZIELD, if you develop liver problems, have a serious infection, or if your blood counts stay too low.
What should I tell my healthcare provider before receiving TZIELD? Before or after receiving TZIELD, tell your healthcare provider about all your medical conditions, including if you:- have a serious infection or an infection that does not go away or keeps coming back
- have recently received or are scheduled to receive an immunization (vaccine). TZIELD may affect how well a vaccine works. Tell your doctor that you are receiving TZIELD before receiving a vaccine
- are pregnant or plan to become pregnant. TZIELD may harm your unborn baby. Do not receive TZIELD during pregnancy and at least 30 days before a planned pregnancy
- are breastfeeding or plan to breastfeed. It is not known if TZIELD passes into your breast milk and if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you receive TZIELD
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of TZIELD? The most common side effects of TZIELD include:- rash
- leukopenia (decrease in white blood cell counts)
- headache
These are not all of the possible side effects of TZIELD. Talk to your healthcare provider for more information, and tell them about any side effects you notice. You may report side effects to the FDA at www.fda.gov/medwatch or 1-800-FDA-1088.
Please see Medication Guide and Prescribing Information.
What is TZIELD?
TZIELD is a prescription medicine used to delay the onset of Stage 3 type 1 diabetes, which is when your body can’t make enough insulin on its own and may require insulin injections. TZIELD is for adults and children 8 years of age and older who have Stage 2 type 1 diabetes. This means that they have tested positive for 2 or more type 1 diabetes-related autoantibodies, have abnormal blood sugar levels, and do not have type 2 diabetes.
It is not known if TZIELD is safe and effective in children under 8 years of age.
Important Safety Information and Approved Use
- Cytokine release syndrome (CRS). Signs and symptoms may start during the first 5 days of TZIELD treatment and could include fever, nausea, feeling tired (fatigue), headache, muscle and joint pain, or increased liver enzymes in your blood. Tell your healthcare provider right away if you develop any signs and symptoms of CRS during treatment with TZIELD
- Decrease in white blood cells. TZIELD may cause a decrease in a type of white blood cell called lymphocytes. A decrease in white blood cells is a serious, but common side effect that can affect your body’s ability to fight infections. A decrease in white blood cell counts can happen after your first dose. Your white blood cell counts will start to go back to normal after your fifth dose of TZIELD. Some people may develop longer and more severe decreases in lymphocytes
Your healthcare provider will do blood tests to check your liver and your complete blood counts before you start treatment and during treatment with TZIELD. During and after your treatment with TZIELD, your healthcare provider will check for serious side effects, as well as other side effects, and treat you as needed. Your healthcare provider may temporarily or completely stop your treatment with TZIELD, if you develop liver problems, have a serious infection, or if your blood counts stay too low.
What should I tell my healthcare provider before receiving TZIELD? Before or after receiving TZIELD, tell your healthcare provider about all your medical conditions, including if you:- have a serious infection or an infection that does not go away or keeps coming back
- have recently received or are scheduled to receive an immunization (vaccine). TZIELD may affect how well a vaccine works. Tell your doctor that you are receiving TZIELD before receiving a vaccine
- are pregnant or plan to become pregnant. TZIELD may harm your unborn baby. Do not receive TZIELD during pregnancy and at least 30 days before a planned pregnancy
- are breastfeeding or plan to breastfeed. It is not known if TZIELD passes into your breast milk and if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you receive TZIELD
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of TZIELD? The most common side effects of TZIELD include:- rash
- leukopenia (decrease in white blood cell counts)
- headache
These are not all of the possible side effects of TZIELD. Talk to your healthcare provider for more information, and tell them about any side effects you notice. You may report side effects to the FDA at www.fda.gov/medwatch or 1-800-FDA-1088.
Please see Medication Guide and Prescribing Information.
What is TZIELD?
TZIELD is a prescription medicine used to delay the onset of Stage 3 type 1 diabetes, which is when your body can’t make enough insulin on its own and may require insulin injections. TZIELD is for adults and children 8 years of age and older who have Stage 2 type 1 diabetes. This means that they have tested positive for 2 or more type 1 diabetes-related autoantibodies, have abnormal blood sugar levels, and do not have type 2 diabetes.
It is not known if TZIELD is safe and effective in children under 8 years of age.